How the Johnson & Johnson vaccine works
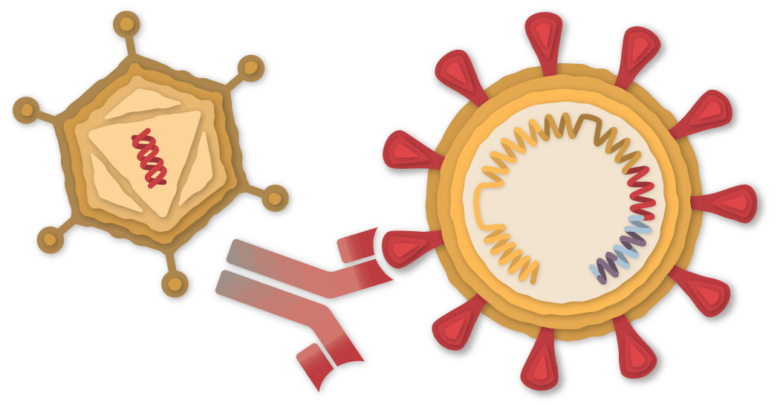

Johnson & Johnson is testing a coronavirus vaccine called JNJ-78436735 or Ad26.COV2.S. Results of a clinical study are expected in January.
Janssen Pharmaceutica, a Belgium-based division of Johnson & Johnson, is developing the vaccine in partnership with Beth Israel Deaconess Medical Center.
A piece of the coronavirus
The SARS-CoV-2 virus is loaded with proteins that it uses to enter human cells. These so-called spike proteins are a tempting target for potential vaccines and treatments.


The Johnson & Johnson vaccine is based on the virus' genetic instructions to build the spike protein. Unlike the Pfizer-BioNTech and Moderna vaccines, which store instructions in single-stranded RNA, the Johnson & Johnson vaccine uses double-stranded DNA.
DNA in an adenovirus
The researchers added the gene for the coronavirus spike protein to another virus called adenovirus 26. Adenoviruses are common viruses that typically cause colds or flu-like symptoms. The Johnson & Johnson team used a modified adenovirus that can enter cells but cannot replicate in cells or cause disease.


Johnson & Johnson's vaccine stems from decades of research into adenovirus-based vaccines. In July the first was approved for general use – an Ebola vaccine, also manufactured by Johnson & Johnson. The company is also conducting trials of adenovirus-based vaccines for other diseases, including H.I.V. and Zika. Some other coronavirus vaccines are also based on adenoviruses, like the one developed by the University of Oxford and AstraZeneca using a chimpanzee adenovirus.
Adenovirus-based vaccines against Covid-19 are more robust than mRNA vaccines from Pfizer and Moderna. DNA isn't as fragile as RNA, and the adenovirus' hard protein shell protects the genetic material inside. As a result, the Johnson & Johnson vaccine can be refrigerated at 2 to 8 ° C for up to three months.
Enter a cell
After the vaccine is injected into a person's arm, the adenoviruses bump into cells and cling to proteins on their surface. The cell swallows the virus into a bubble and pulls it inside. Inside, the adenovirus escapes from the bladder and migrates to the nucleus, the chamber in which the cell's DNA is stored.

Virus devoured
in a bubble

Virus devoured
in a bubble

Virus devoured
in a bubble





The adenovirus pushes its DNA into the nucleus. The adenovirus is designed so that it cannot make copies of itself, but the gene for the coronavirus spike protein can be read by the cell and copied into a molecule called messenger RNA or mRNA.
Structure of spike proteins
The mRNA leaves the nucleus and the cell's molecules read their sequence and start building spike proteins.

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments

Three spines
Proteins combine
spikes
and protein
Fragments
Show
Spike protein
Fragments
Some of the spike proteins produced by the cell form spikes that migrate to its surface and their tips stick out. The vaccinated cells also break down into fragments some of the proteins that they present on their surface. These protruding spikes and spike protein fragments can then be recognized by the immune system.
The adenovirus also provokes the immune system by turning on the cell's alarm systems. The cell sends out warning signals to activate nearby immune cells. By triggering this alarm, the Johnson & Johnson vaccine makes the immune system more responsive to the spike proteins.
Discover the intruder
When a vaccinated cell dies, the debris contains spike proteins and protein fragments, which can then be taken up by a type of immune cell called an antigen-presenting cell.

Present a
Spike protein
fragment

Present a
Spike protein
fragment

Present a
Spike protein
fragment
The cell presents fragments of the spike protein on its surface. When other cells called helper T cells recognize these fragments, the helper T cells can set off the alarm and help other immune cells fight the infection.
Make antibodies
Other immune cells, called B cells, can bump against the coronavirus spikes on the surface of vaccinated cells or against free-floating spike protein fragments. Some of the B cells may be able to bind to the spike proteins. When these B cells are then activated by helper T cells, they begin to multiply and pour out antibodies that target the spike protein.

Matching
Surface proteins

Matching
Surface proteins

Matching
Surface proteins

Matching
Surface proteins

Matching
Surface proteins

Matching
Surface proteins

Matching
surface
Proteins

Matching
surface
Proteins

Matching
surface
Proteins

Matching
Surface proteins

Matching
Surface proteins

Matching
Surface proteins
Stop the virus
The antibodies can attach to coronavirus spikes, mark the virus for destruction, and prevent infection by preventing the spikes from attaching to other cells.
Kill infected cells
The antigen presenting cells can also activate another type of immune cell called a killer T cell to search for and destroy any coronavirus infected cells that have the spike protein fragments on their surfaces.

Present a
Spike protein
fragment
Beginning
to kill them
infected cell

Present a
Spike protein
fragment
Beginning
to kill them
infected cell

Present a
Spike protein
fragment
Beginning
to kill them
infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell

Present a
Spike protein
fragment
I'm starting to kill
the infected cell
Memory of the virus
Johnson & Johnson is testing a single dose of the vaccine, unlike the coronavirus vaccines with two doses from Pfizer, Moderna and AstraZeneca. Because the results of the Johnson & Johnson Phase 3 study have not yet been published, the researchers have no idea how well the vaccine might work or how long it might last to protect.
If the vaccine is effective, it is possible that antibody and killer T-cell counts may decrease in the months following vaccination. The immune system also contains special cells, so-called storage B cells and storage T cells, which can store information about the coronavirus for years or even decades.
Vaccination schedule
January 2020 Johnson & Johnson begins work on a coronavirus vaccine.
March Johnson & Johnson will receive $ 456 million from the US government to develop and manufacture the vaccine.
July A phase 1/2 study begins. Unlike the clinical trials for other leading vaccines, the trial is one dose, not two.
![]()
A dose of the Johnson & Johnson vaccine.Michael Ciaglo / Getty Images
August The federal government agrees to pay Johnson & Johnson $ 1 billion for 100 million doses if the vaccine is approved.
September Johnson & Johnson starts a phase 3 study.
8th October The European Union reaches an agreement to obtain 200 million cans.
12th of October The company is suspending its phase 3 study to investigate a side effect in a volunteer.
23rd October The process continues.
November 16 Johnson & Johnson announces a second Phase 3 study to evaluate the effects of two doses of their vaccine instead of just one.
December 17th Johnson & Johnson announces that the phase 3 study with approximately 45,000 participants is fully enrolled.
January 2021 Preliminary results of the phase 3 study are expected in January. The company aims to produce at least one billion cans by 2021.
February If studies show the vaccine is effective, the company could apply for an emergency clearance with the Food and Drug Administration in February.
Sources: National Center for Information on Biotechnology; Nature; Lynda Coughlan, University of Maryland Medical School.